Brand name medications are often scrutinized by the workers’ compensation industry for their high cost. When there is a generic available, pharmacies should dispense the generic. However, when a generic substitute does not exist, what other alternatives are available for the workers’ compensation industry? One option is to use therapeutic alternatives. This is not alternative medicine. Rather, therapeutic alternatives, or therapeutic equivalents, are made of different chemicals than the brand name drug, but “can be expected to have [nearly] the same clinical effect and safety profile when administered to patients under the conditions specified in the labeling.” This drug update will cover the basics of therapeutic alternatives and provide some examples relevant to the workers’ compensation and auto casualty industries.
Generic Medications vs. Therapeutic Alternatives
Two main questions arise when discussing alternatives to brand-name medications: What are therapeutic alternatives? How do they differ from generic drugs? Let’s first look at generic drugs. According to the Federal Drug Administration (FDA), “A generic drug is a medication created to be the same as an existing approved brand-name drug in dosage form, safety, strength, route of administration, quality, and performance characteristics.” The FDA emphasizes that generic drugs work in the same way as brand drugs, providing the same clinical benefit. When a generic is not available as a substitute for a brand name drug, prescribers and pharmacists may consider therapeutic alternatives, where appropriate, to provide a lower-cost, equally efficacious option to patients. Let’s now look at some key therapeutic alternatives.
Therapeutic Alternatives in the Workers’ Compensation and Auto Casualty Industries
All medications and their therapeutic alternatives are listed in equivalent dosing. All of these dosings equate to roughly a 30-day supply.
Multi-Ingredient Drugs
This is one area of opportunity to consider in your program. Multi-ingredient drugs are often espoused as a way to help reduce the number of pills that a patient has to take. Although this could be beneficial for certain patients, for the most part, there is little clinical advantage of taking one pill versus two. The difference lies in the cost of the drugs. Multi-ingredient drugs often cost far more than their individual ingredients, and provide the same therapeutic benefit. Vimovo and Duexis are two key examples frequently seen in the workers’ compensation and auto casualty industries.
Vimovo
Vimovo contains naproxen 500mg and esomeprazole (generic Nexium) 20mg. Naproxen is an anti-inflammatory and esomeprazole is used to prevent gastric upset caused by anti-inflammatory medications. 
Duexis
Duexis contains ibuprofen 800mg and famotidine 26.6mg. Similar to Vimovo, ibuprofen is a non-steroidal anti-inflammatory (NSAID) and famotidine is an H2 blocker, used to prevent gastric upset caused by the NSAID. Since famotidine (Pepcid) does not come in a 26.6mg strength, we are comparing this to the usual dose of 20mg. 
Special Dosage Forms
Abuse-deterrent formulations (ADF’s) are another area that could be considered for therapeutic alternatives. ADF’s are intended to make manipulation of the product difficult, preventing patients from turning it in to an immediate release drug (e.g. by crushing, cutting or grinding the capsule). These formulations were specifically designed to prevent abuse, particularly in opioids. While ADF’s are certainly beneficial for some patients, not all patients require them. When evaluating these prescriptions, consider your claimant’s medication history along with their overall recovery to determine if further evaluation of therapeutic alternatives is beneficial. One example is:
Morphabond ER
Morphabond ER is an extended release formulation of morphine, manufactured as an ADF. According to the FDA, Morphabond is approved for treatment of “pain severe enough to require daily, around-the-clock, long-term opioid treatment.” 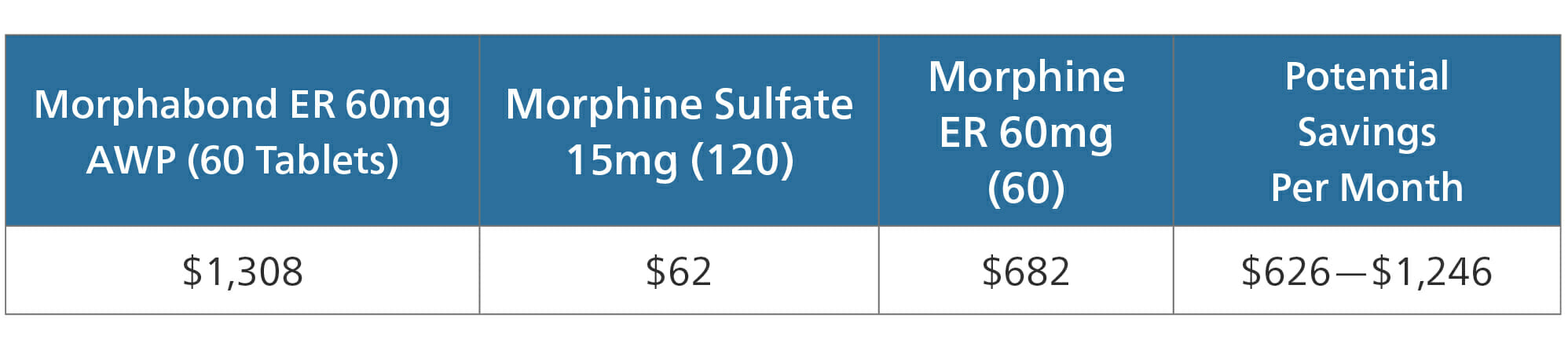
Compounding Kits
Compounding kits are on the rise in many different therapeutic categories. A compounding kit typically consists of a powder and base to mix into a cream. Similar to multi-ingredient medications, compounding kits have therapeutic alternatives that may be slightly different in how they are applied (e.g. gel versus cream), but provide the same therapeutic benefit at a lower cost.
EnovaRX-diclofenac
EnovaRX-diclofenac consists of a kit containing the ingredients for a pharmacist to mix diclofenac powder into a cream. This drug and its alternatives are used for pain therapy. Diclofenac is manufactured in a gel. Although these two products have a different base (gel versus cream), both are applied topically and contain the same drug ingredient, diclofenac. 
Strengths
Strength differences in medications are an important issue to watch in your pharmacy program. Some manufacturers produce an existing generic drug in a different strength and patent it, which then allows that drug to be marketed at a higher price.
Lidocaine
A major example of this type of therapeutic alternative is the drug lidocaine, specifically patches containing lidocaine, plus or minus other ingredients. Lidocaine patches are used to relieve pain in localized areas, usually back, knees or shoulder. Lidocaine patches are available by prescription at 5% strength. However, a 4% lidocaine patch is available over-the-counter.
- The AWP for 30 lidocaine 5% patches is $308
- The AWP for 30 lidocaine 4% patches is $45
Several other patch types follow similar markups. 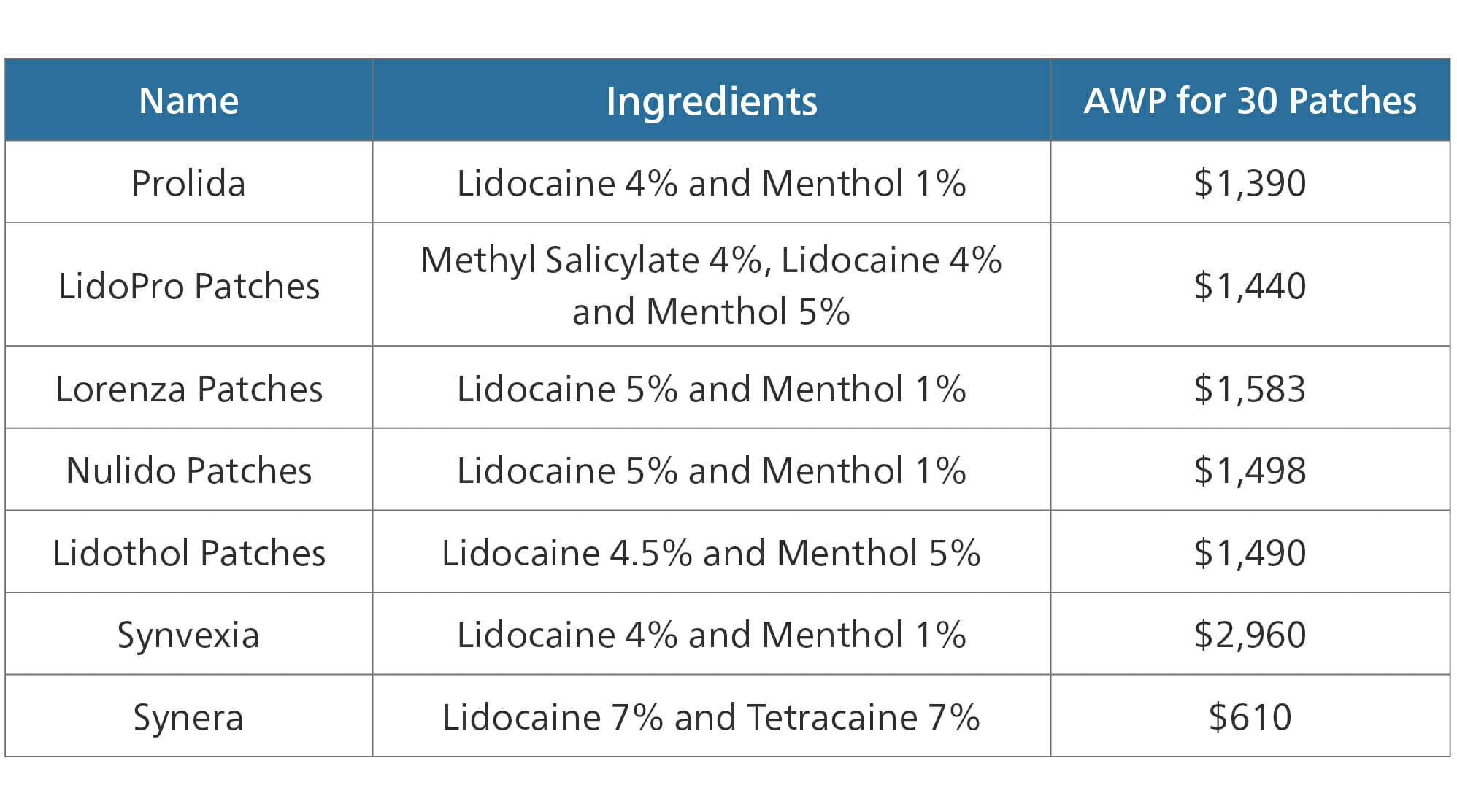
Xtampza ER
Xtampza ER is another example where strength is used as a differentiator. Xtampza ER is oxycodone extended release in an abuse-deterrent formulation. Xtampza ER is manufactured in 9mg, 13.5mg, 18mg, 27mg and 36mg capsules. Its therapeutic alternative, oxycodone ER generic is manufactured in 10mg, 15mg, 20mg, 30mg, 40mg, 60mg and 80mg tablets. A 30-day supply of Xtampza ER 18mg has an AWP of $658, while a 30-day supply of Oxycodone ER 20mg has an AWP of $277. Per the Xtampza ER manufacture’s package insert, the strengths of Xtampza ER are all equivalent to one of the strengths of oxycodone ER generic (e.g. 9mg of Xtampza ER is equivalent to 10mg oxycodone ER, 13.5mg Xtampza ER is equivalent to 15mg oxycodone ER, 18mg Xtampza ER is equivalent to 20mg oxycodone ER, etc.). Unless an abuse-deterrent formulation is required, generic formulations are acceptable therapeutic alternatives.
Extended Release Formulations
Another great example of a therapeutic alternative are extended release formulations. These formulations are typically prescribed so that a patient can take the drug fewer times per day. Most extended release formulations are 12-hour or 24-hour capsules or tablets, whereas regular release medications are typically taken every 4-6 hours. Besides the length of time between doses, the extended and regular release formulations offer similar therapeutic benefits.
Amrix
Amrix is cyclobenzaprine extended release capsules. Cyclobenzaprine is a muscle relaxant. 
Trokendi XR
Trokendi XR is an extended release formulation of topiramate. Topiramate is an anti-seizure medication that is also used to treat migraines. 
How to Manage Brands and Therapeutic Alternatives
There are many different types of therapeutic alternatives, which can be an excellent option when a generic for a specific drug is not available. Although not appropriate in every case, it is important to identify when therapeutic alternatives can be used when a brand name drug is prescribed. Work with your Pharmacy Benefit Management (PBM) team to evaluate your formularies and determine when therapeutic alternatives can and should be used.


