State-Mandated Formulary Implementation: Four Key Considerations
As more states move to create drug formulary rules, how can you best prepare your business for formulary implementation?
The state-mandated formulary wave is poised to continue in 2019. In just the first month of the year, an additional three states published draft or final formulary rules (New York, Montana and Kentucky). These laws provide guidance on the prescribing of medications to injured workers and are effective in addressing major workers’ compensation issues. As this trend continues, how can you best prepare your company for the new requirements? This article will take you through some major considerations for new state-mandated formulary implementation, from understanding the intricacies of formularies to asking the right questions for your business.
1. Understand Formulary Basics
A basic formulary is a list of preferred medications that physicians are able to prescribe and pharmacies are able to dispense without prior authorization. If a medication is appropriate for the injury, proven to be effective, low risk and low cost, then it will automatically be approved. Medications or combinations of medications that do not fall under these requirements will require additional authorization before they can be dispensed. State-mandated formularies provide overarching guidance for prescribing in that state and can work alongside formularies designed by payors and PBM’s to provide the best outcomes for injured workers. In some cases, formularies can be straightforward, but in many cases, they are very complex. The next section will cover these complexities and look closely at California formulary implementation.
2. Understand Unique Requirements in Each State
Although the basics of each formulary are similar, every state can develop specific rules that are unique to its jurisdiction. Texas was an early adopter and showed early positive results, setting the stage for other states to adopt formularies. As of the beginning of 2019, nine states have fully implemented drug formularies and three more have posted draft formulary rules specific to the workers’ compensation industry. Some have followed Texas in the adoption of the ODG Formulary, while others, such as California, have adopted a formulary more consistent with the ACOEM guidelines. A few states, like Washington, have developed their own, custom formulary that applies to the workers’ compensation industry.
Since formularies are complex, it is important to understand not only the basics, but also how each state approaches factors like prior authorization. California is a great example of this complexity, since the formulary requires further level of scrutiny before allowing the fill or sending the fill to prior-authorization, by looking at the drug itself as well as the drug in context with the injury. The purpose is to ensure that the medication is not only safe but also appropriate for the worker’s injury.
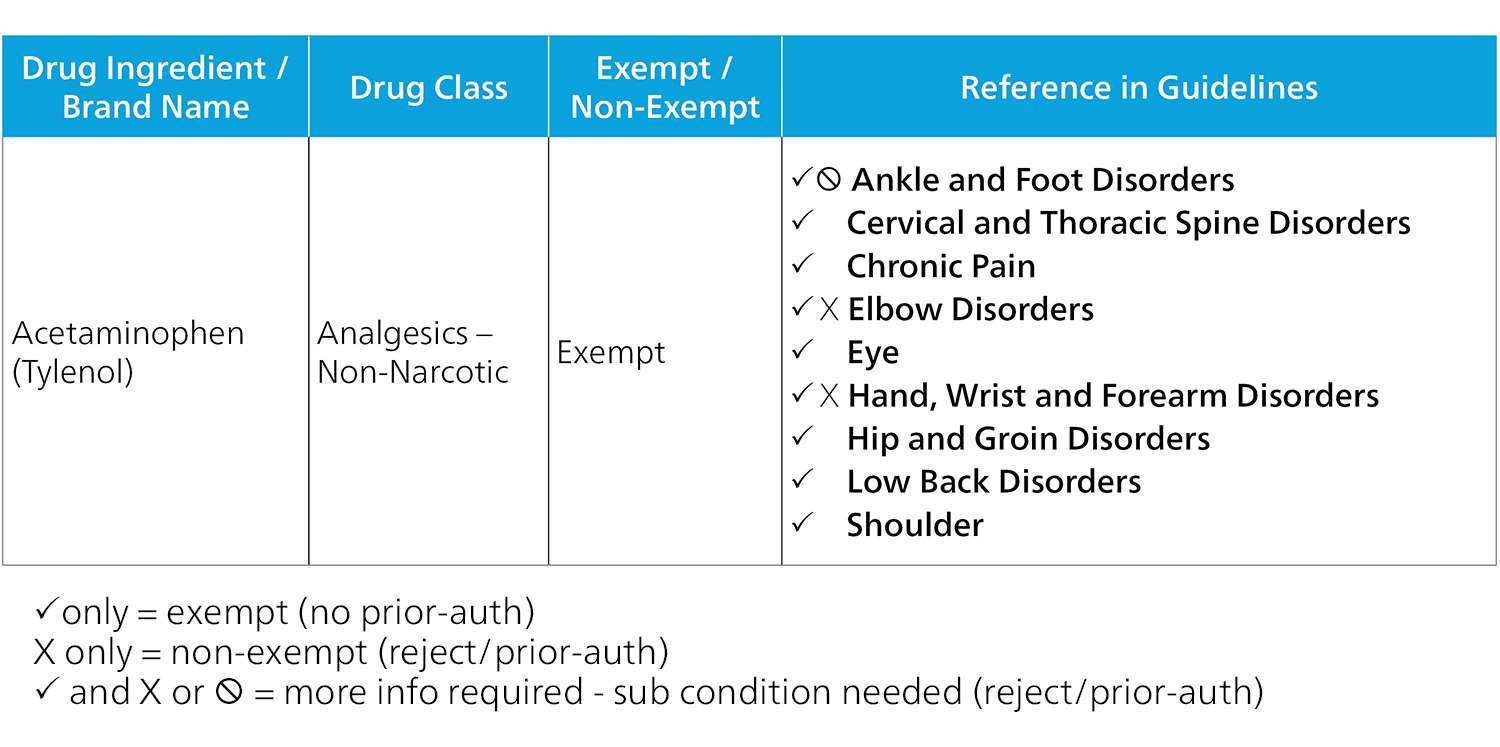
Exempt Example
Beyond this, states may have differing requirements for compounds, first fills and legacy claims, among others. It is important to understand these complexities and differences to approach formulary implementation and apply the necessary controls correctly. For instance, Tylenol will automatically be approved for a shoulder injury but will require more information for a wrist injury. Depending on the type of wrist injury, the medication may be approved without review or may need to go to utilization review. We will look more closely at this level of intricacy in utilization review in the upcoming sections.
3. Impact on Your Business
When a state decides to go down the formulary road, they typically begin with draft legislation where they solicit public input for review of the legislation. This is an excellent time to make any recommendations that you believe will produce the best outcomes for all parties. Then, once all inputs are considered, the formulary is released along with an implementation or go-live date. Frequently, this is six months or further in the future. There are many considerations to take into account during this period between publication and go-live, including:
- What is the implementation date for the formulary and when do all claims need to be switched over to the new formulary?
- What is the state’s policy for legacy claims and how can you best prepare your claimants? (Often legacy claims have a grace period before formulary rules apply to them.)
- What is the state’s policy for utilization review and how does its integration with the new formulary affect your business processes? What changes might you need to make to your workflow or staffing levels in the short and long term? (See the next section for more.)
- What kinds of medications (e.g. opioids or compounds) are included on the mandatory review list?
- Do you need to update your current formulary to be compliant? If any updates need to be made, make sure to work closely with your PBM to ensure compliance.
- Do you have the right data in your system for proper formulary implementation? For example, the California formulary is based on the body part injury level.
- What communications have you prepared? To workers? Physicians? Pharmacies? Employers?
Importantly, state-mandated formularies can be implemented differently by various PBM’s and businesses, which will affect your business processes. One major consideration is how utilization review is handled when dealing with complex formulary implementation. This is addressed next.
4. Formulary and Utilization Review
Many states have mandatory utilization review (UR), which is defined as “a critical evaluation (as by a physician or nurse) of health-care services provided to patients that are made especially for the purpose of controlling costs and monitoring quality of care.” When treatment is in question, it must be sent to utilization review to make a determination of whether or not it should be allowed. When implementing a state-mandated formulary with mandatory utilization review, it is important to find a balance between minimizing utilization review costs and implementing good clinical control. If a state has existing mandatory UR review and implements a formulary, there can sometimes be an unexpected spike in UR costs. This initially may seem like a negative outcome, but there are other aspects to consider as well.
Changes in Prescribing Habits
Initial formulary implementation may see more drugs sent to utilization review, but after some time, UR rates will likely fall. This occurs for several reasons. Because formularies put more scrutiny on the prescribed drugs, prescribers begin to focus on prescriptions for medications that are on-formulary and ultimately more appropriate for the injured worker. Over time, this can result in decreased UR costs, since there are fewer medications going to UR. For instance, between Texas’s formulary implementation in 2011 and 2014, 85 percent fewer injured workers in the state were prescribed drugs that required prior authorization and associated costs decreased by 80 percent. Similarly, Ohio saw prescribing costs decrease by $47 million between 2011 and 2017, with a $24 million decrease in opioid spending. Additionally, Texas and Ohio have both seen decreases in opioid utilization since implementing their formularies, with opioid costs dropping from 27 percent to 18 percent in Texas and opioid dependence decreasing by 59% in Ohio. These examples show how formularies can be a great driver of positive change in a state’s workers’ compensation system.
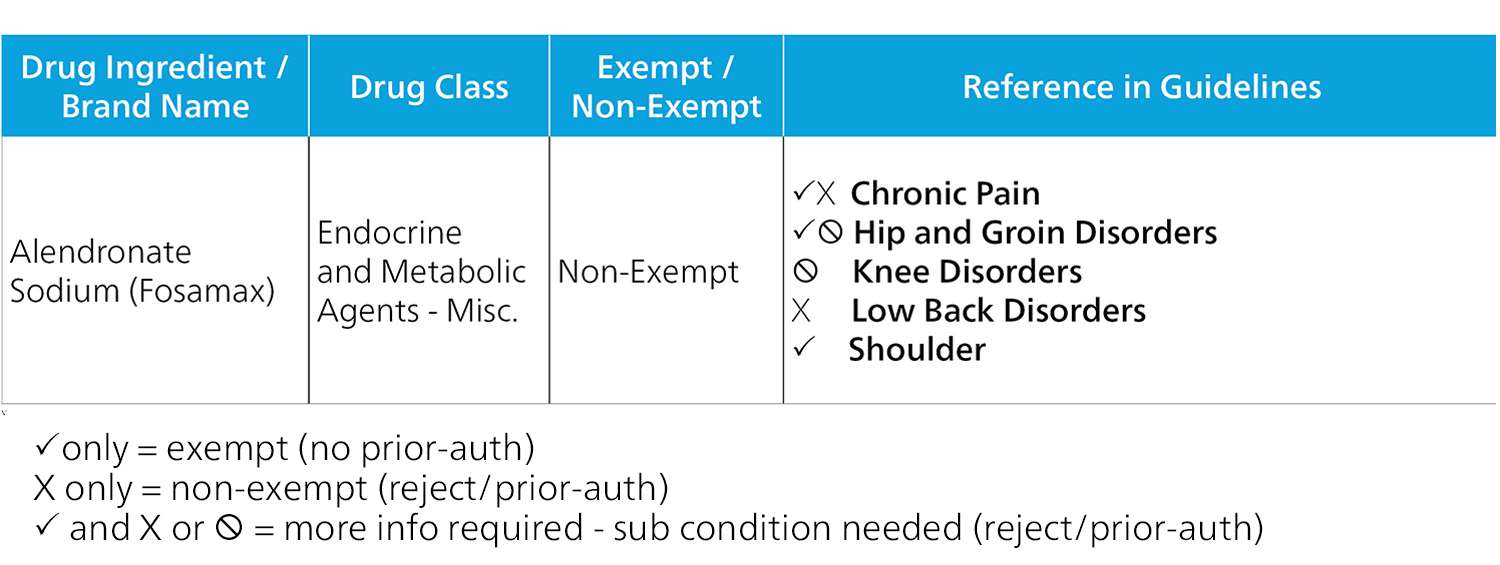
Complexity of Formulary
As addressed earlier, each state’s formulary has its own complexities. How the formulary is implemented can also affect deployment of utilization review and associated costs. In the case of the California formulary, if a PBM only implements the formulary at the exempt and non-exempt level, certain drugs may be sent to UR even if the injury does not require UR. For instance, the California formulary classifies Fosamax as a non-exempt drug, which requires UR in and of itself. However, if an injured worker is prescribed Fosamax for a shoulder injury, the drug falls into the exempt category and can be dispensed without utilization review.
Non-Exempt Example
On the other end of the spectrum, Tylenol falls into the exempt category. However, there are some injury types that are non-exempt for Tylenol and do require utilization review. The drug may be appropriate for some injuries but not others. This complexity is overlooked if the formulary is only implemented on the exempt and non-exempt level. To complicate matters further, payers may decide that not all drugs requiring UR should be sent to UR. For example, while Tylenol is not indicated for some specific injuries, its low risk and low cost may not warrant an expensive UR. Because California only requires that PBM formularies not be more restrictive than the California formulary, PBM’s technically only need to implement the formulary up to the exempt and non-exempt level. The additional complexity, however, provides that the injured worker is receiving the most appropriate care and that your business is only sending medications to UR when necessary. Consider how the formularies in the state you operate are affecting your business processes this way and work with your PBM partner to implement the best way possible.
Appeals Process
Another issue that may arise from formulary implementation is whether the state has a robust UR process in place. If a state has a mandatory formulary but not mandatory UR, the appeals process can be more challenging and ultimately disruptive to the injured workers’ recovery. The consideration here is whether a business chooses to handle the process up front by implementing its own UR requirements or on the back end with an appeals process. If a state does not have a formulary, businesses and claimants will typically have to go through an appeals process. The ultimate takeaway here is that every state and every payer are different. The formulary process and its effects can be very complex and have a large impact on your business. It is important to have a business partner that understands the complexities as well as your business in order to implement in a way that is best for your company and the injured workers it serves.
What to Look for in 2019
As we head into the first few months of 2019, we expect a few states to be looking at formulary legislation. Montana just published its final formulary rule while Kentucky and New York recently posted their draft formulary rules. We expect Nebraska and a few other states to look at formulary creation this year. As the industry prepares for these upcoming formularies, it is important to consider how these multifaceted changes can affect outcomes and businesses processes both positively and negatively. Every state is different, which means that having a partner that understands the nuances and complexities of the formulary as well as how to implement it well is critical to ensure best outcomes.