Nearly 20 percent of U.S. adults suffer from chronic pain. This is especially vital in the workers’ compensation world that is now asking, ‘are gabapentinoids the answer to that pain?’ Current increases in prescribing of gabapentinoids and the drugs’ less addictive qualities as compared to opioids may initially suggest so. However, recent research and legislative changes at the state level suggest that, in the push to find alternatives to opioids, we may not be finding the right answer in gabapentinoids. Several questions arise as we move forward in trying to find alternative ways to manage pain. Are gabapentinoids effective at treating pain? Are they a viable alternative? Are they the answer to the opioid crisis? Let’s take a closer look at these medications and their use in workers’ compensation, including considerations we should make as we help get injured workers back to health.
Background on Gabapentinoids
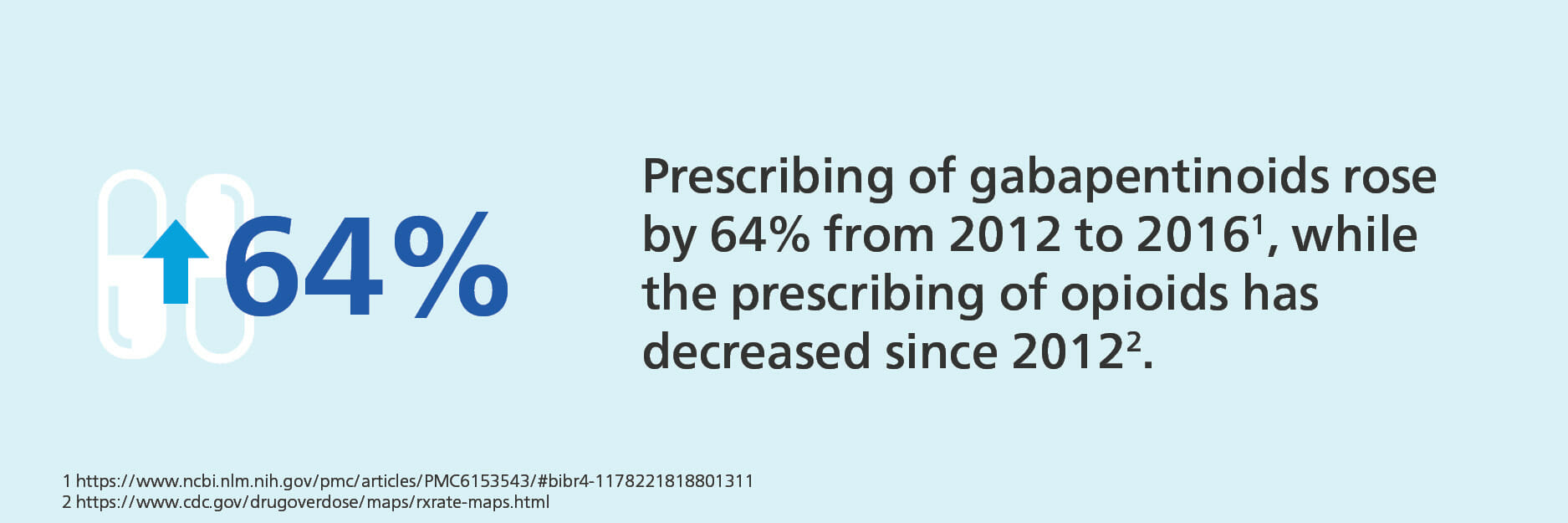
Gabapentinoids are a sub-class of anticonvulsant medications, which includes gabapentin (brand name Neurontin) and pregabalin (brand name Lyrica). Both have been available on the market for more than a decade, with gabapentin earning FDA approval in 1993 and pregabalin approved in 2004. This class of drugs is approved for treatment of epilepsy, postherpetic neuralgia, neuropathic pain and fibromyalgia, among other conditions. (View Lyrica’s indications and usage here. View Neurontin’s indications and usage here.) According to NCCI, Lyrica was the number one drug, in terms of cost, prescribed in the workers’ compensation industry in 2016. In July 2019, the first generic version of pregabalin became available, which may help reduce some of the cost associated with the brand name version. With gabapentinoids’ ability to treat neuropathic pain, some have considered the drug class an optional alternative for opioids. However, the use of gabapentinoids for off-label pain treatment is becoming increasingly common. In fact, the FDA commissioner said in a speech in 2018 that, “Some literature suggests that clinicians may be prescribing these drugs off-label to patients with various types of pain, as alternatives to opioids, outside the approved indications for gabapentinoids.” Some studies have found that up to 95 percent of gabapentin is prescribed today for off-label indications. Prescribing of gabapentinoids has also risen in recent years, by 64% from 2012 to 2016. Opioid prescribing, meanwhile, has decreased since its peak in 2012.
The CDC, in its opioid prescribing guidelines study, states, “Several guidelines agree that first- and second-line drugs for neuropathic pain include anticonvulsants (gabapentin or pregabalin)….” The guidelines do not suggest uses outside of the FDA’s indicated uses, but do suggest that gabapentinoids are a good option to try to treat neuropathic pain before opioids are attempted. As we better understand the increase in prescribing of gabapentinoids, especially for off-label use, what should we consider for the workers’ compensation industry?
Gabapentinoids' Use in Workers’ Compensation
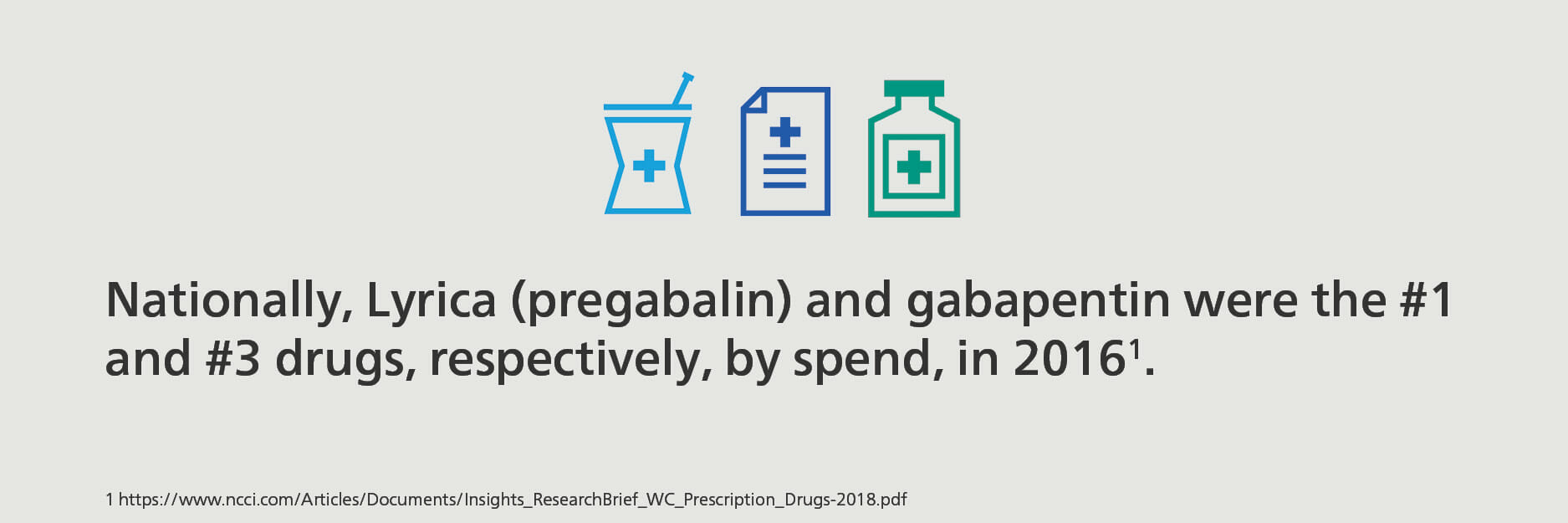
In workers’ compensation, gabapentinoids are most often used for the treatment of neuropathic pain associated with spinal cord injury or other areas of the body where nerves may have been damaged by an injury and cause substantial pain for the injured worker. The trend in decreased opioid prescribing and increased gapabentin and pregabalin prescribing is similar in workers’ compensation. In 2018, anticonvulsants became the third most prescribed therapeutic classification of medications in the California workers’ compensation space, based on a study by CWCI. Nationally, Lyrica (pregabalin) and gabapentin were the #1 and #3 drugs, respectively, by spend, in 2016, making up 7.2% and 3.8% of the total drug spend in the workers’ compensation arena.
Does this increase in prescribing mean that gabapentinoids are a good alternative for treating pain? Not necessarily.
Risks of Gabapentinoids
As the rate of gabapentinoid prescribing increases, it is important to consider the risks associated with the drugs. Several studies have found that gabapentinoid abuse is rising, especially in conjunction with opioid abuse. In a 2017 review of 59 studies, researchers from the University of Texas et al. found that “gabapentinoids possess potential for abuse, particularly in individuals with a history of opioid abuse, and reports of such abuse are increasingly being documented.” In fact, a 2017 study from Midwestern University found that “gabapentin use patterns are similar to those of other abusable medications.” The use of gabapentinoids in conjunction with opioids is of particular concern, since the overuse of gabapentin along with opioids has been shown to increase the risk of respiratory depression by four times. A study from Canada found that “moderate-dose and high-dose gabapentin use was associated with a nearly 60% increase in the odds of opioid-related death relative to no concomitant gabapentin use.” In December 2019, the FDA released a warning about serious breathing problems associated with gabapentinoids when using opioids or other drugs that depress the central nervous system, or for people with chronic obstructive pulmonary disease (COPD).
Regulations on Gabapentinoids: A New Trend?
As more becomes known about gabapentinoids, their side effects and their potential for abuse, the federal government and a few states have taken action to control the spread of gabapentinoid abuse. Gabapentin and pregabalin are both non-exempt on the California MTUS drug formulary, and require prior authorization for specific body parts. Gabapentin’s use is limited to a 4 days’ supply. ODG, on the other hand, categorizes gabapentin and pregabalin as ‘Y’ drugs, which means they are a part of the ODG formulary. In 2005, the DEA reclassified pregabalin (Lyrica) as a Schedule V controlled substance. As such, the drug is considered to:
- Have a low potential for abuse relative to drugs in Schedule IV.
- Have a currently accepted medical use.
- Abuse of the drug may lead to limited physical dependence or psychological dependence relative to the drugs in Schedule IV.
For reference, most opioids, including oxycodone and hydrocodone, are listed as Schedule II drugs. Gabapentin has not been scheduled as a controlled substance by the federal government. Five states, however, list gabapentin as a controlled substance, including Kentucky, Michigan, Tennessee, Virginia and West Virginia.
Interestingly, in July 2019, North Dakota published and implemented dose limits for pregabalin (Lyrica) for all existing and new prescriptions. The new limits took effect August 15, 2019. The limits for Lyrica are three capsules per day for the 25, 50, 75, and 100 mg strengths, two capsules per day for the 150, 200, 225, and 300 mg strengths, and one capsule per day for the 82.5, 165, and 330mg controlled release strengths per day. The dosage limits in North Dakota translate to a maximum daily dose of 600 mg (two capsules of the 300mg strength). Six hundred milligrams per day is also the maximum dose recommended by Pfizer for Lyrica. Whether this becomes a trend as more states begin to examine the potential downsides to gabapentinoids remains to be seen. As we continue to fight the opioid crisis, we must be vigilant in studying alternatives and their role in pain management and helping injured workers return to health.