It seems there is always something new to say when it comes to the hot topic of medical marijuana or “cannabis,” as it’s commonly referred to in clinical literature. But while the cannabis regulatory landscape seems to continually change and evolve, scientific evidence has moved much more slowly. And when it comes to medicine, evidence matters. The subject of cannabis for medicinal use is unique in that it is one of those rare occurrences where policy has jumped ahead of science. In other words, federal and state regulators have been pushed into making decisions related to legality and use of medical cannabis in advance of having a sound body of reliable, large-scale scientific research that establishes clear guidance around the safety and efficacy of such products.
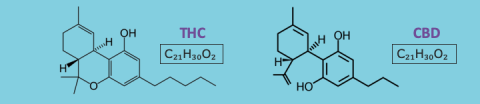
When we’re talking about cannabis products, it’s important to understand where they come from and how they work. Both “marijuana” for recreational and medicinal use and “hemp” are derived from the cannabis plant. The active compounds of the plant that are responsible for its effects are known as “cannabinoids,” and the two with the most therapeutic interest are THC (△9-tetrahydrocannabinol) and CBD (cannabidiol). Upon introduction to the human body, these compounds activate various receptors that lead to a variety of responses, including anti-inflammatory, analgesic, anti-nausea, and anti-seizure effects, to name a few. The full effects on the human body remain largely unknown, but we do know that cannabinoids can exhibit clinically-meaningful drug-drug and/or drug-food interactions and can be associated with a number of unwanted cardiovascular, respiratory, psychoactive, gastrointestinal, and neurological effects.
Notably, THC is responsible for the “high” associated with cannabis use, while CBD is not. Largely for this reason, CBD has become the subject of much study and can be found virtually everywhere in today’s marketplace with various health claims ranging from pain management to better sleep to relief from everyday stresses/anxiety to overall general wellness. However, these products, with the exception of prescription CBD Epidiolex®, do not follow the traditional drug approval process under the U.S. Food and Drug Administration (FDA), and therefore have not been evaluated by the FDA for safety and efficacy. Navigating product selection can be difficult for this reason necessitating a “buyer beware” mentality considering issues such as product inconsistency, quality and purity unknowns, packaging and labeling discrepancies, improper testing, lack of dosing guidance, and misconstrued marketing or advertising claims.
From a clinical perspective, cannabis and its derivatives may have a potential role to play in a variety of conditions. However, given today’s cannabis environment, limitations of current medical cannabis research should be carefully and critically evaluated and weighed addressing individual patient health concerns; misconceptions; procurement and regulatory challenges; availability of guideline-supported, FDA-approved treatment alternatives, and an overall focus on risk vs. benefit.
For additional insights on medical marijuana and cannabis use, including regulatory updates and perspectives, join us next week for our annual webinar series Medical Marijuana Highs & Lows: Work Comp Clinical and Regulatory Trends. Details and registration on our website.


